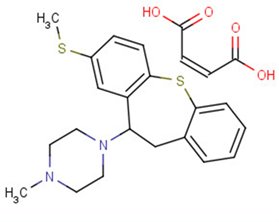
Methiothepin maleate
CAS No. 19728-88-2
Methiothepin maleate ( Methiothepin Maleate | Metitepine | HCVIN281816 )
产品货号. M18192 CAS No. 19728-88-2
Methiothepin 是一种 5-HT1、5-HT6、5-HT7 血清素受体拮抗剂,可阻断血清素自身受体。
纯度: >98% (HPLC)
 COA
COA
 Datasheet
Datasheet
 HNMR
HNMR
 HPLC
HPLC
 MSDS
MSDS
 Handing Instructions
Handing Instructions
| 规格 | 价格/人民币 | 库存 | 数量 |
| 50MG | ¥502 | 有现货 |


|
| 100MG | ¥915 | 有现货 |


|
| 500MG | ¥3426 | 有现货 |


|
| 1G | 获取报价 | 有现货 |


|
生物学信息
-
产品名称Methiothepin maleate
-
注意事项本公司产品仅用于科研实验,不得用于人体或动物的临床与诊断
-
产品简述Methiothepin 是一种 5-HT1、5-HT6、5-HT7 血清素受体拮抗剂,可阻断血清素自身受体。
-
产品描述Methiothepin Maleate is an inhibitor of HCV infection and cell-to-cell transmission that acts by targeting the HCV E2 glycoprotein.
-
体外实验Methiothepin maleate is a 5-HT receptor antagonist, with pKds of 7.10, 7.28, 7.56, and 6.99 for 5-HT1A, 5HT1B, 5HT1C, 5HT1D. Methiothepin mesylate also shows pKds of 7.0, 7.8, 8.74, and 8.99 for 5-HT5A, 5-HT5B, 5-HT6, and 5-HT7, respectively. Methiothepin exhibits high affinity at 5-HT2A, 5HT2B, and 5HT2C with pKis of 8.50, 8.68, and 8.35, respectively.
-
体内实验——
-
同义词Methiothepin Maleate | Metitepine | HCVIN281816
-
通路Others
-
靶点Other Targets
-
受体5-HT1|5-HT6|5-HT7
-
研究领域——
-
适应症——
化学信息
-
CAS Number19728-88-2
-
分子量472.62
-
分子式C24H28N2O4S2
-
纯度>98% (HPLC)
-
溶解度——
-
SMILESCN1CCN(CC1)C2CC3=CC=CC=C3SC4=C2C=C(C=C4)SC.C(=CC(=O)O)C(=O)O
-
化学全称1-Methyl-4-(3-methylsulfanyl-5,6-dihydrobenzo[b][1]benzothiepin-5-yl)piperazine Maleate
运输与储存
-
储存条件(-20℃)
-
运输条件With Ice Pack
-
稳定性≥ 2 years
参考文献
1. Knight J A, etal. Molecular Pharmacology, 2009, 75(2):374-380.



 021-51111890
021-51111890 购物车()
购物车()
 sales@molnova.cn
sales@molnova.cn











